Through the eyes of our clients
We grow by creating added value!
We produce works that comply with the legislation with
🗸 our global and local pharmacovigilance solutions,
🗸 effective communication,
🗸 quality-oriented services,
🗸 administrative authority approved projects
01234567890012345678900123456789001234567890 +
M o l e c u l e s
We provide service globally with our pharmacovigilance solutions.
01234567890012345678900123456789001234567890 +
P r o d u c t s
Pharmacovigilance Department services / Regulatory Affairs Department services
01234567890012345678900123456789001234567890 +
P r o j e c t s
Pharmacovigilance, regulatory affairs, IT projects
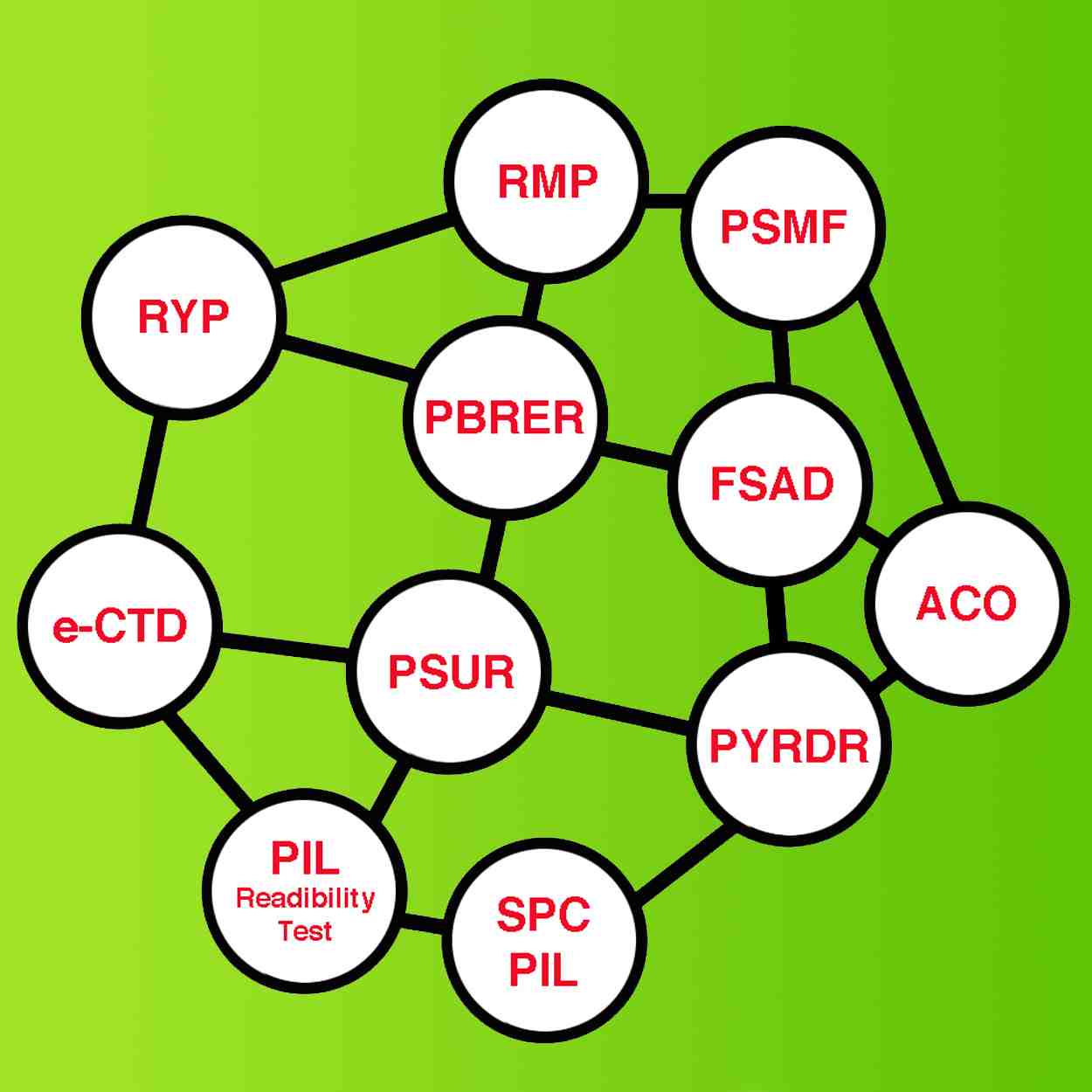
Facilitate your procedures with our
Agile Cooperation & Team of Experts & Outstanding Technology,
Global & Local
Compliance with local and global administrative standards such as TMMDA (Turkish Medicines and Medical Devices Agency), EMA and FDA
Effective Communication
Instant feedback to all your requests within maximum 24 hours
Measurable
Measurable Key Performance Indicators with PharmaBASE Compliance Management Module
End-to-end transparent process
With a cloud-based access, enjoy the opportunity to see your works are completed.











































